1.2 Heat transfer
In the previous example heat was transferred via conduction, one of the three fundamental modes of heat transfer. These are:
1) Conduction
A group of molecules vibrating with more energy (thus, higher temperature) interact with nearby slower-moving molecules and transfer some of that kinetic energy. This direct transfer of kinetic energy is heat conduction. Conduction continues in a body until the kinetic vibrational energy is evenly distributed, i.e. in equilibrium. In gases, conduction is caused by the intermolecular collisions and diffusion of molecules during their random motion.

2) Convection
A group of molecules with a certain thermal energy can be physically moved to a different location (e.g., the flow of a hot fluid). Therefore an energy flow has taken place. This is convection. Often a flowing fluid absorbs heat (via very local conduction) from a warmer body and then transports it via convection to a colder body elsewhere.
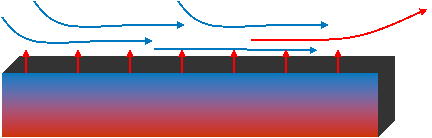
3) Radiation
All objects constantly emit and absorb photons. Each photon has a certain energy. When a photon is emitted, the body loses that amount of energy, and when a photon is absorbed, the body gains that energy. The warmer an object is, the more energetic the photons that it emits will be. Therefore, a warm body in radiative contact next to cold body loses thermal energy on average, because it emits more energy than it absorbs from the cold body. The warmer body gets colder and the cold body gets warmer, until their temperatures are equal. At that point they each absorb and emit equal amounts of energy.
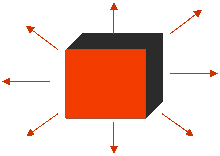
These three modes of heat transfer are explained in more detail in the following sections.
